| Atmosphere Chemistry: Ozonolysis Reactions
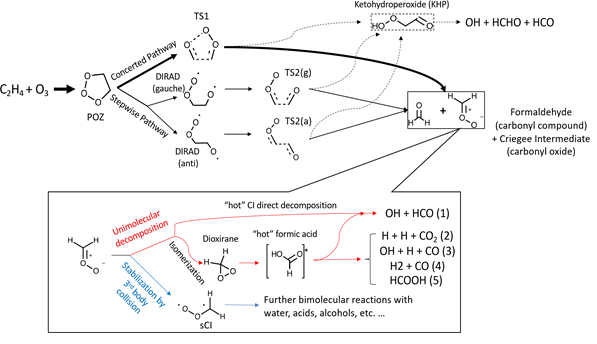
In this program, the ozonolysis reaction of ethylene (C2H4+O3) is studied experimentally in a flow reactor coupled with photoionization mass spectrometer. New products and intermediates from ozonolysis reactions are detected and confirmed by quantum chemistry calculations. Those new species include C2H2, CH3CH2OH, ethen-1,2-diol, methyl formate, etc. In addition, the secondary ozonide, i.e. 1,2,4-trioxolane is identified experimentally. Some small hyperoxides and peroxyl radicals are confirmed as intermediates which are H2O2, CH3OOH, CH2CHOO, C2H5OO.
|
| Ozone Assisted Combustion
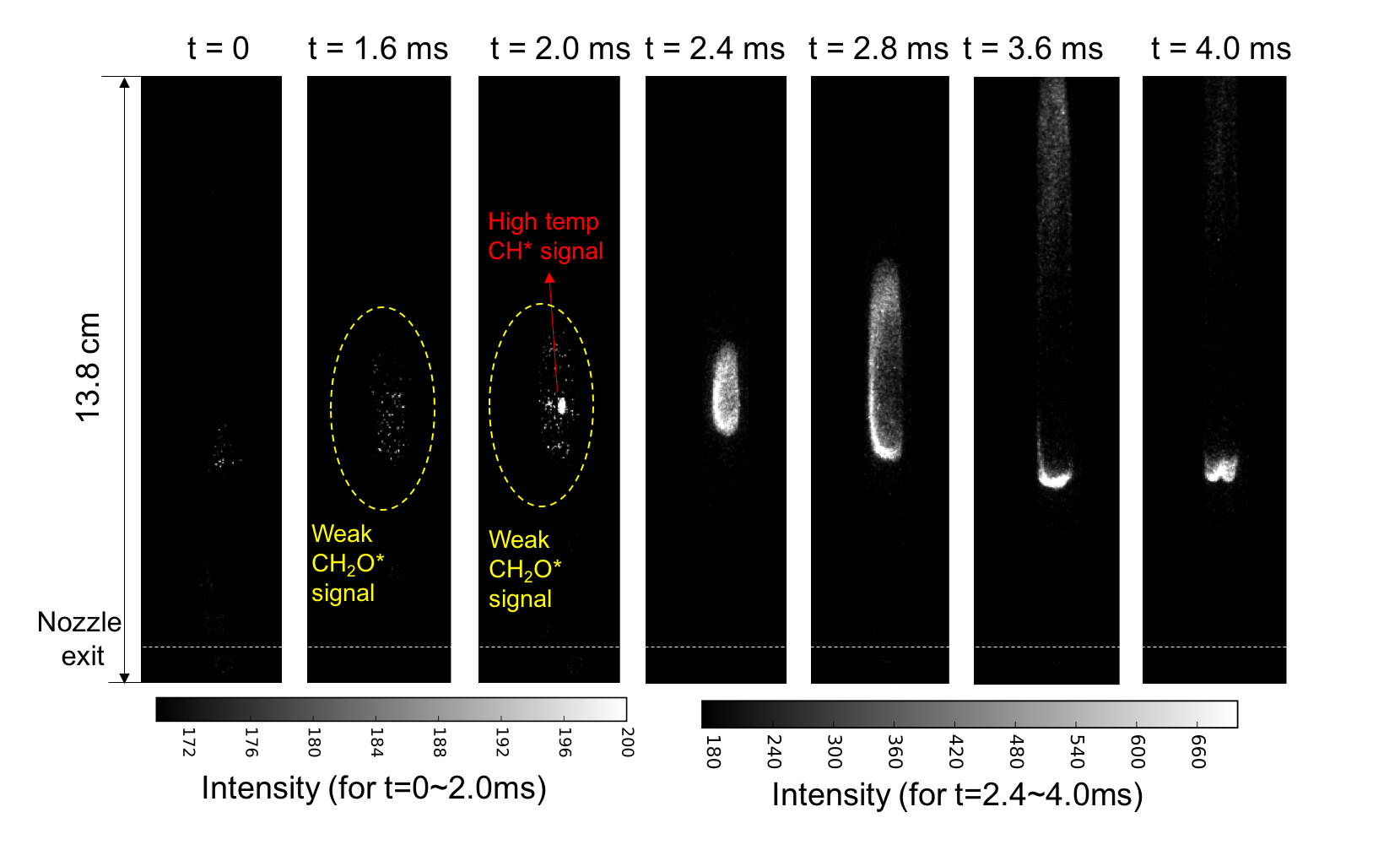
Plasma/ozone-assisted combustion is a promising technique to improve engine performance, increase lean burn flame stability, reduce emissions, and enhance low temperature fuel oxidation and processing. Plasma/ozone-assisted combustion takes advantage of the dramatically different kinetics between plasma/ozone and combustion to enhance and control the combustion process. We work on using plasma generated species to enhance and control combustion process. One ongoing project is to study the effect of ozone addition on combustion. One well known reaction pathway to enhance combustion by ozone addition through ozone decomposition (O3àO2+O). Different from conventional understanding in which ozone was known to enhance flame speeds owing to its unique capability to release atomic oxygen (O3àO+O2) at elevated temperature conditions, We also discovered that ozone (through ozonolysis reactions) can induce explosive reactions at extremely low temperature conditions (even at room temperature) in combustion systems with unsaturated hydrocarbons, such as ethylene. It is common sense that combustion only occurs at high temperature conditions and can only be initiated by ignitors producing a high temperature environment. However, by taking advantage of ozonolysis reactions, (e.g., C2H4+O3àCH2O+H2+CO2, typically studied in atmosphere chemistry community regarding ozone layer depletion and pollution) autoignition was demonstrated at room temperature conditions. A new autoignition-assisted flame stabilization mechanism was also atreported by us. Our work on ozone-enhanced combustion bridges the study in the atmosphere chemistry community and in the combustion community. It will provide a solution to enable low-temperature combustion and combustion at near limiting conditions for the development of advanced engines. This research also has the potential to develop a new aerated fuel injection technique and a fuel coking removal technique, therefore changing the cycle efficiency. In this experiment, ozone is doped into synthetic air and ethylene is used as fuel to create autoigniting environment in diffusion jet flame. the flame dynamics of autoigniting flame is investigated. Figure below shows high speed images right after the fuel jet was turned on. Ozonolysis reactions between O3 and C2H4 produce large amount of CH2O and release heat. The chemiluminescence measurement indicates the formation of a cloud of CH2O, then an auignition kernel occured inside the CH2O cloud. In such a environment, flame could propagate orders of magnitude faster than its corresponding laminar flame speed.
|
| High Pressure Combustion Kinetics
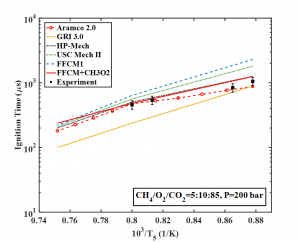
Recently, we developed a new and unique high pressure shock tube to study high pressure combustion kinetics. It enables measurement of critical fundamental combustion parameters in a completely new pressure region, especially those associated with combustion at a supercritical carbon dioxide (sCO2) condition suitable for a future power generation system. This is a regime where combustion kinetics has never been explored before. The sCO2 power cycle has higher efficiency and allows almost 100% carbon capture with no NOx emission (Zero Carbon Natural Gas, selected as 10 breakthroughs technologies in 2018 by MIT Technology Review). Once successful, this technique will potentially change the landscape of ground power generation. However, the sCO2 power cycle requires the combustor to run in the pressure range of 100 atm to 300 atm with high CO2 concentration, which is completely different from the operating regime of conventional gas turbines. For the first time in this field, we obtained autoignition delay of CH4/O2/CO2 autoignition delays at 100±7 atm at the sCO2 condition as shown in the plot below. Comparison with selected kinetic models shows that GRI 3.0 which is widely used in industries has been proven to have large deviation from experiments at sCO2 condition. |
| Combustion Instability Control Using Plasma
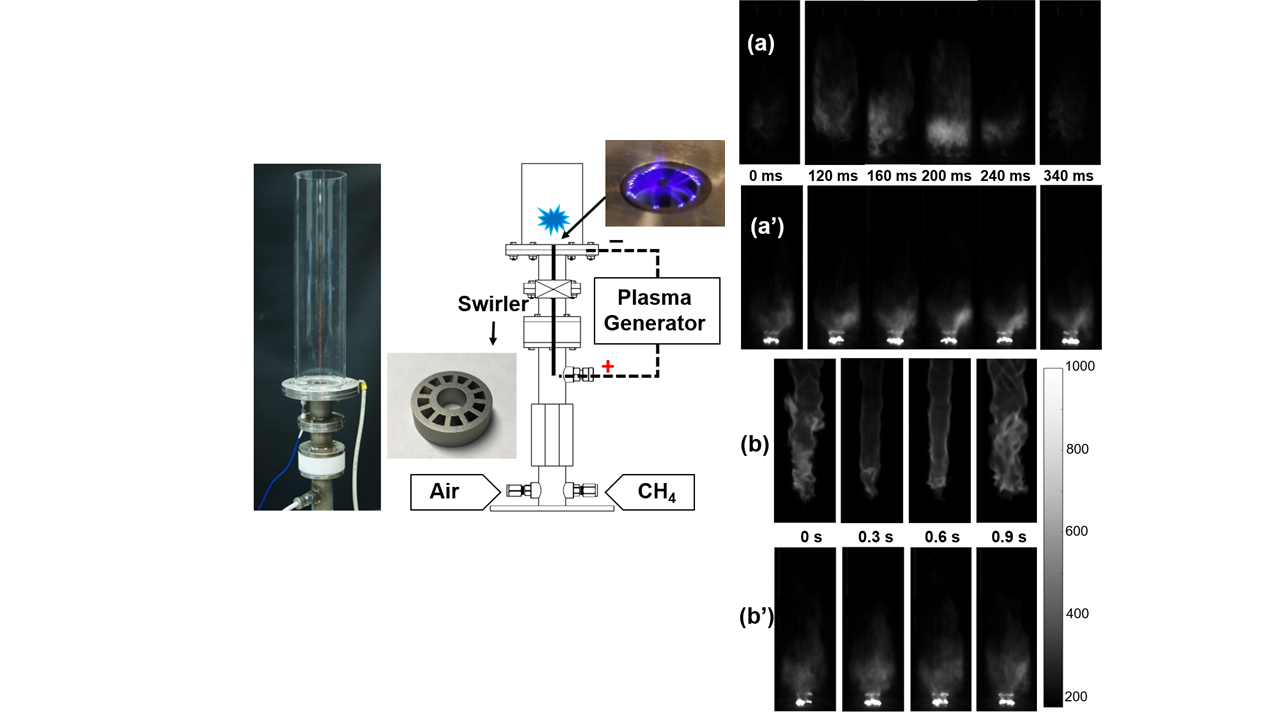
In this project, we use non-equilibrium nanosecond pulsed plasma to control combustion instability. At a condition close to lean blowoff, flame starts to oscillate (a) and finally blows off with further decrease of equivalence ratio. With plasma activation, the lean blowoff limits can be significantly extended and flame can be stablized without osscilation (a’). At certain conditions, plasma also change the morphology of flames (b) and (b’).
|
|
Kinetic Model Reduction and Dynamic Adaptive Kinetics for Turbulent Combustion
|